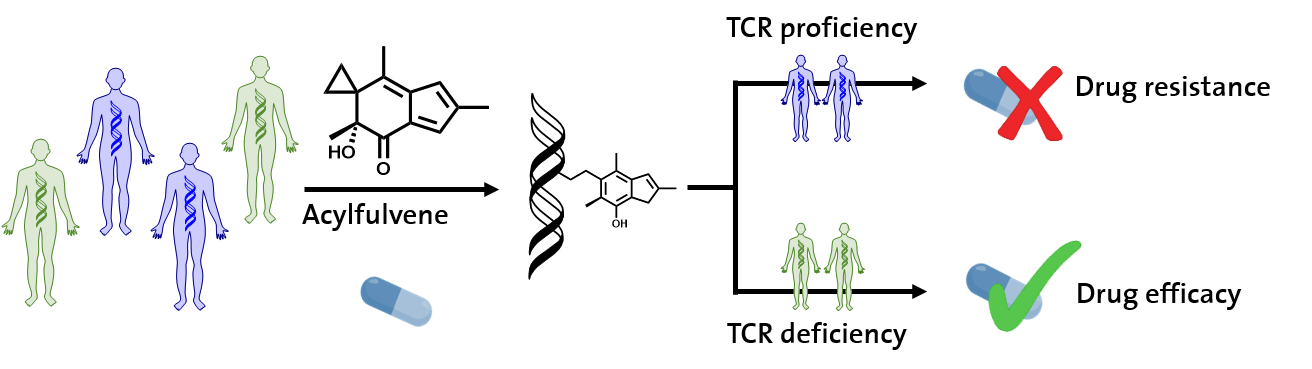Hijacking Transcription-Coupled DNA Repair for Cancer Therapy
There has been great progress in cancer therapy in the past decades, including the more effective use of drugs that damage DNA of cancer cells; however, an active DNA damage response in these cells remains a major factor for drug resistance. Experimental anticancer acylfulvenes (AF) are a class of reductase-activated DNA alkylating pro-drugs with favorable tumor selectivity profiles. Over the past 15 years, our group has uncovered a molecular basis for tumor cell selectivity of these agents based on elucidating mechanisms of bioactivation and DNA alkylation, and have devised novel strategies for selective sensitization of cells by activation of metabolism, as well as bioanalytical approaches for predicting drug sensitivity. More recently, we have focused on transcription coupled-nucleotide excision repair (TC-NER) as a basis of drug resistance. In detailed chemical and biochemical studies, we have gained deep insight into how minor groove AF-DNA adducts interfere with the progress of RNA polymerase II and initiate repair. These results suggest new directions for exploiting the metabolic requirements and selective repair profiles of AF to devise new agents or combinations that target the molecular characteristics of cancers that are resistant to AF or current standard therapies.
Representative Publications
Malvezzi, S., Farnung, L., Aloisi, C.M.N, Angelov, T., Cramer, P., Sturla, S.J., (2017) Mechanism of RNA polymerase II stalling by DNA alkylation, PNAS, 114 (46), 12172-12177
external page https://doi.org/10.1073/pnas.1706592114
Otto, C., Spivak, G., Aloisi, C.M.N, Menigatti M., Naegeli H., Hanawalt P.C., Tanasova M., Sturla S.J., (2017) Modulation of Cytotoxicity by Transcription-Coupled Nucleotide Excision Repair Is Independent of the Requirement for Bioactivation of Acylfulvene, Chem Res. Toxicol., 30 (3), 769-776
external page https://doi.org/10.1021/acs.chemrestox.6b00240
Tanasova, M., Sturla, S.J., (2012) Chemistry and Biology of Acylfulvenes: Sesquiterpene-Derived Antitumor Agents, Chem. Rev, 112 (6), 3578-3610
external page https://doi.org/10.1021/cr2001367
Collaboration & Funding Partners
Rubin Lab
Department for Biomedical Research, University of Bern, Switzerland
external page https://rubinlab.squarespace.com/
Schäerer Lab
Institute for Basic Science - Center for Genomic Integrity, Ulsan National Institute of Science and Technology, Republic of Korea
external page https://www.scharerlaboratory.org/