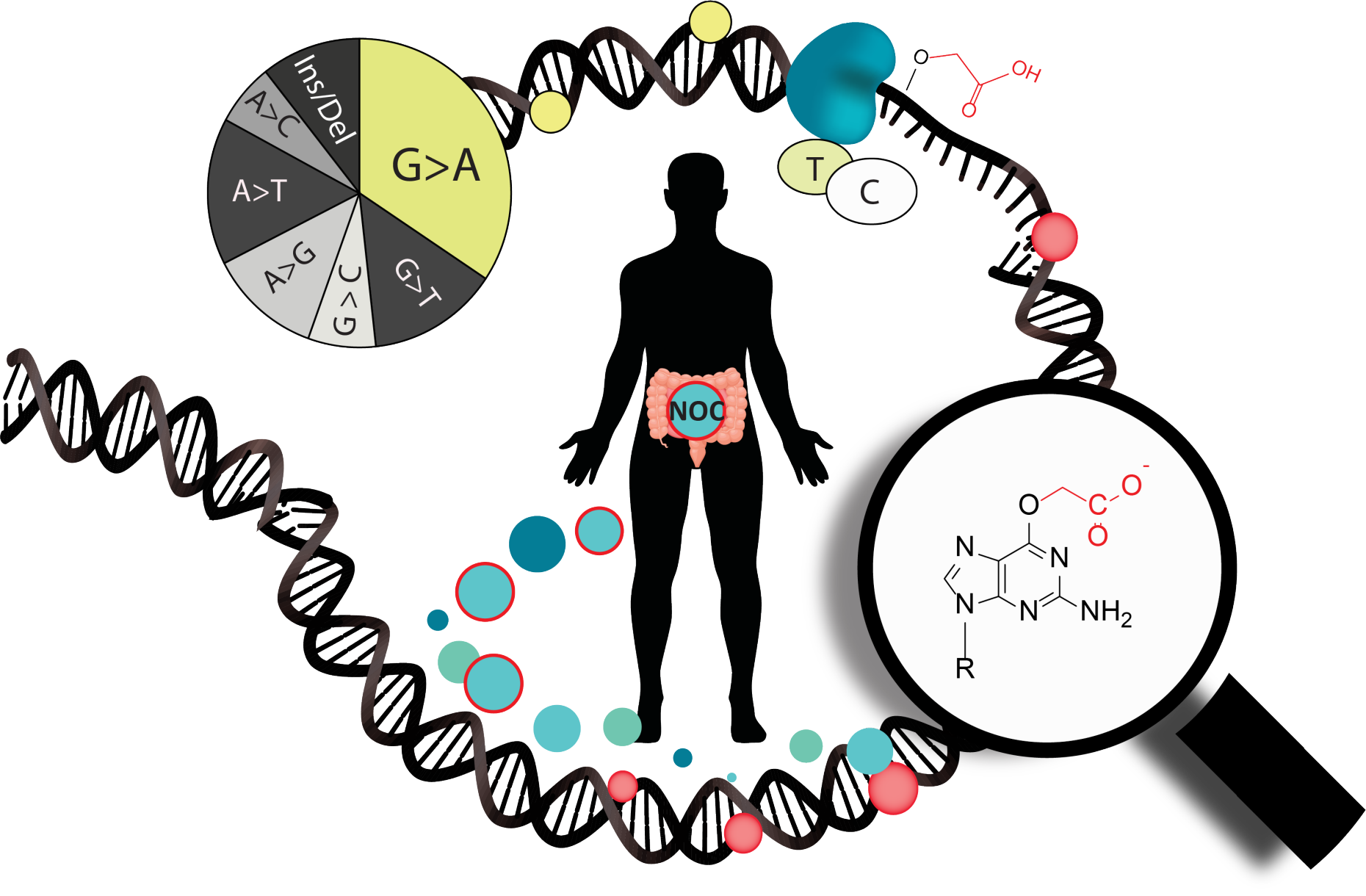
Mutagenicity of O6-Carboxymethylated DNA
O6carboxymethylguanine (O6-CMG) is a DNA adduct that arises from the nitrosation of amino acids and is associated with high red and processed meat consumption. Our goal is to understand factors governing the formation, distribution, and persistence of O6-CMG in DNA, and to elucidate mechanisms by which it can lead to mutations driving colorectal carcinogenesis. Our studies have combined chemical synthesis, biochemical assays and bioanalytical chemistry to elucidate how O6-CMG can promote base misincorporation during DNA replication and to take advantage of the efficient insertion of artificial nucleotides opposite O6-CMG in order to identify, locate and quantify this mutagenic DNA adduct. Our recent work concerning how O6-CMG is repaired in cells suggested a potential cross-talk between direct reversion and nucleotide excision repair, and we aim to determine how accessibility to nucleosomal DNA impacts O6-CMG repair. We anticipate that a deepened understanding of the chemical and molecular basis of O6-CMG formation, repair and mutagenicity can enable future cancer prevention strategies that integrate biomarkers of individual cancer risk.
Representative Publications
Aloisi, Claudia M.N., Escher, Nora A., Kim, Hyun S., Geisen, Susanne M., Fontana, Gabriele A., Yeo, Jung-Eun, Schärer, Orlando D., Sturla, Shana J., (2022). A combination of direct reversion and nucleotide excision repair counters the mutagenic effects of DNA carboxymethylation, DNA Repair 110, 103262, 1568-7864
external page https://www.sciencedirect.com/science/article/pii/S1568786421002184
Geisen, Susanne M., Aloisi, Claudia M. N., Huber, Sabrina M., Sandell, Emma S., Escher, Nora A., Sturla, Shana J. (2021). Direct Alkylation of Deoxyguanosine by Azaserine Leads to O6-Carboxymethyldeoxyguanosine. Chemical Research in Toxicology 34.6, 1518-1529
external page https://pubs.acs.org/doi/10.1021/acs.chemrestox.0c00471
Aloisi Claudia M.N., Sandell Emma S., Sturla Shana J, (2021). A Chemical Link between Meat Consumption and Colorectal Cancer Development? Chemical Research in Toxicology 18;34(1):12-23
external page https://pubs.acs.org/doi/pdf/10.1021/acs.chemrestox.0c00395
Aloisi, Claudia M. N., Nilforoushan, Arman, Ziegler, Nathalie, Sturla, Shana J., (2020). Sequence-Specific Quantitation of Mutagenic DNA Damage via Polymerase Amplification with an Artificial Nucleotide. Journal of American Chemical Society 142.15, 6962-6969
https://pubs.acs.org/doi/10.1021/jacs.9b11746
Räz, M. H., Sandell, E., Patil, K., Gillingham, D. G., and Sturla, S. J. (2019). High Sensitivity of Human Translesion DNA Synthesis Polymerase κ to Variation in O6-carboxymethylguanine Structures. ACS Chemical Biology, 14.2, 214-222
external page https://pubs.acs.org/doi/10.1021/acschembio.8b00802
Geigle, S. N., Wyss, L. A., Sturla, S. J., Gillingham, D. G. (2017). Copper carbenes alkylate guanine chemoselectively through a substrate directed reaction. Chemical Science, 8 (1) 499-506.
external page https://pubs.rsc.org/en/content/articlelanding/2017/sc/c6sc03502g
Räz, M. H., Dexter, H. R., Millington, C. L., Loon, B., Williams, D. M., and Sturla, S. J. (2016). Bypass of mutagenic O6-Carboxymethylguanine DNA adducts by human Y- and B-Family polymerases. Chemical Research in Toxicology 29.9, 1493-1503
external page https://pubs.acs.org/doi/10.1021/acs.chemrestox.6b00168